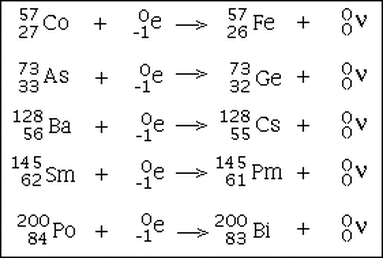
Some examples of electron capture. The electron disappears and a new neutron is created from a proton combining with the electron. A neutrino is given off, along with X-rays which are the result of photons being given off as outer shell electrons drop down to lower energy levels to fill the gap and give off energy.
