There are four naturally occuring decay series on earth (there were more but the initial isotopes have all been used up as their half lives were shorter than the age of the earth). They are Uranium-238 to Lead-206,
Uranium-235 to Lead-207, Thorium-232 to Lead-208 and Neptunium-237 to Bismuth-209. The short half life of Neptunium-237 (2.14 Million years) means it no longer exists naturally, but most of the rest of the decay series continues today. (this decay series is known because Neptunium-237 is created in some atomic reactors, and by decay of Uranium)
Uranium-235 to Lead-207, Thorium-232 to Lead-208 and Neptunium-237 to Bismuth-209. The short half life of Neptunium-237 (2.14 Million years) means it no longer exists naturally, but most of the rest of the decay series continues today. (this decay series is known because Neptunium-237 is created in some atomic reactors, and by decay of Uranium)
Binding Energy and Nuclear Forces
The total mass of a stable nucleus is always less than the sum of the masses of the protons and neutrons in the nucleus. This is because the lost mass has been converted to energy (Einstein suggested that mass might be considered to be a form of energy, convertible to other forms of energy such as Kinetic, Electrical etc). This energy is referred to as the Total Binding Energy and is equal to the energy required to break the nucleons apart.
There is more than one force acting in the nucleus, and the reason the nucleons stay together is due to a force that is very strongly attractive at very small distances, called the Strong Nuclear Force. It is very strong between nucleons if they are less than about 10^-15 metres apart, beyond this it is essentially zero.
This force has some strange quirks, and is not fully understood. There is also a Weak Nuclear Force, and these two, along with the Electromagnetic Force and Gravitational force make up the four known types of force in nature.
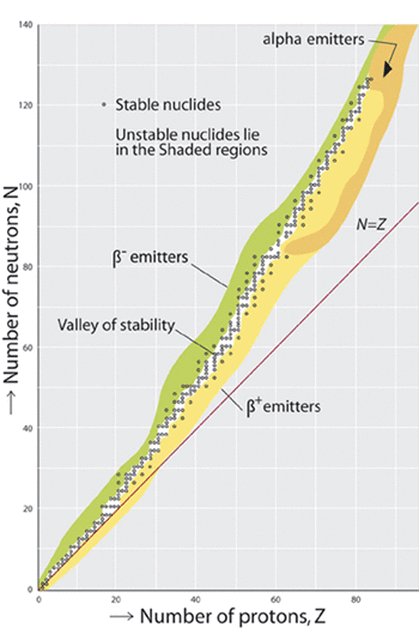
If the nuclide has too many or too few neutrons relative to the number of protons, the binding of the nucleons is reduced and the nucleus is unstable, meaning it comes apart, resulting in radioactive decay. Up to an atomic number of about 30-40, the stable nuclei tend to have close to the same number of protons and neutrons. Above this, stable nuclei contain more neutrons than protons. This is becasue the electrical repulsion between the protons increases with more protons, so more neutrons are needed to exert the attractive nuclear force. A point is reached where no number of neutrons can overcome the increasing electrical repulsion, and all nuclides with atomic number greater than Z=82(Lead) are unstable.
This force has some strange quirks, and is not fully understood. There is also a Weak Nuclear Force, and these two, along with the Electromagnetic Force and Gravitational force make up the four known types of force in nature.
If the nuclide has too many or too few neutrons relative to the number of protons, the binding of the nucleons is reduced and the nucleus is unstable, meaning it comes apart, resulting in radioactive decay. Up to an atomic number of about 30-40, the stable nuclei tend to have close to the same number of protons and neutrons. Above this, stable nuclei contain more neutrons than protons. This is becasue the electrical repulsion between the protons increases with more protons, so more neutrons are needed to exert the attractive nuclear force. A point is reached where no number of neutrons can overcome the increasing electrical repulsion, and all nuclides with atomic number greater than Z=82(Lead) are unstable.